Andreas Beuchel

photo: Andreas Beuchel
May 2020;
source: private
Infections caused by non-tuberculous mycobacteria (NTM) pose a serious threat for patients with suppressed immune system or preexisting lung conditions. NTMs include all mycobacteria that do not cause tuberculosis (Mycobacterium tuberculosis, Mtb) or leprosis (Mycobacterium leprae). Infections with NTMs lead to damage of the lung tissue and severe breathing problems. An estimated 50% of the predisposed patients are infected with NTMs. The most prevalent species are the Mycobacterium abscessus-complex (MABC) and the Mycobacterium avium-complex (MAC). Although related to Mtb, NTMs demonstrate different levels of resistance to first-line drugs against tuberculosis. Mycobacterium abscessus (Mab) in particular is known as one of the most resistant bacterium to antiinfectives. In addition the efficacy of therapeutics, e.g. clarithromycin, is often affected by biofilm formation of Mab or a decrease of activity is observed during therapy due to inducible drug resistance. Currently there is no medicine approved to cure NTM infections and only a few substances are in clinical trials. SPR720, a DNA gyrase inhibitor developed by Spero Therapeutics, is the most promising candidate for a new treatment option (clinical phase 1).
We established cooperation between our lab and the Center for Discovery and Innovation (CDI) of the Hackensack Meridian Health (HMH) (Nutley, New Jersey, USA) with the objective to develop novel substances to fight NTM infections. About 20 hits were identified from an initial screening of open source compound libraries which contained substances against neglected infectious diseases including tuberculosis. One of them is MMV688844, a piperidin-4-carboxamide that shows bactericidal activity against both Mtb and Mab (Figure 1). In literature MMV688844 is described as an inhibitor of a mycobacterial ABC-transporter. Due to the fact that this target has been identified through in silico analysis, further investigation is needed.
Structure of MMV688844
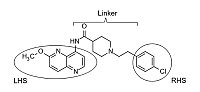
The molecule consists of three building blocks: a methoxy-substituted aminonaphthyrdine on the left-hand side (LHS), which is connected to piperidin-4-carboxylic acid via amide bond (linker). The right-hand side is a para-chloro-substituted phenyl moiety linked to the piperidin by two methylene groups (RHS).
source: private
My research is focused on the synthesis and characterization of novel MMV688844 analogues including their microbiological evaluation. My core competence is the planning and implementation of synthetic sequences, reaction work up and analytical characterization of small molecules. In cooperation with the CDI, promising candidates were identified and continuously improved through the methods of medicinal chemistry. This includes both the pharmacodynamic and pharmacokinetic attributes of the substances in which in vitro activity and stability assays are involved. In addition, I modulate the physicochemical properties of the candidates in order to improve their in vivo pharmacokinetics. Critical parameter is solubility and permeability of test compounds. In case one of both parameters is too low the bioavailability in the infected tissue (lungs) will not reach sufficient levels. Subsequently, the efficacy is determined in an infected mouse model, in order to investigate the in vivo activity.
In addition to drug discovery and development, I am also interested in the identification of molecular targets and the biochemical processes that are responsible for the activity of the molecules. In cooperation with the lab of Prof. Sippl, we take advantage of the knowledge for in silico analysis and structure optimization at MLU to predict molecules. Subsequently the synthesis of predicted test compounds is planned and carried out by me. Eventually, the structures are tested for their microbiological activity in the labs at the CDI and the MLU.




